Heidelberg, Germany, May 6, 2025 Heidelberg Epignostix GmbH, a deeptech start-up committed to precision cancer diagnostics, announces today that the Centers for Medicare & Medicaid Services (CMS) have released a preliminary Medicare reimbursement rate for the CPT® (Current Procedural Technology) code for the Company’s proprietary Epignostix CNS Tumor Methylation Classifier – a molecular test for precision diagnostics of tumors of the central nervous system. The code completed the gapfill payment determination process, following CMS’ November 2024 recommendation. The code, 0020M, is described as “Oncology (central nervous system), analysis of 30000 DNA methylation loci by methylation array, utilizing DNA extracted from tumor tissue, diagnostic algorithm reported as probability of matching a reference tumor subclass”.
CPT codes are used by medical practitioners, including physicians, laboratories, and other healthcare providers, to report their services to payers and to seek reimbursement. This standardized national system of identification provides a uniform language for reporting healthcare procedures and services. The proposed Medicare reimbursement rate for the Epignostix CNS Tumor Methylation Classifier is released for comment before finalization in July and will be effective January 2026.
The company has established methylation-based precision tumor classification due to the high need for improved diagnostic accuracy across all fields of oncology. It is already recommended by WHO guidelines as a precision classification tool for brain tumors, enabling matching therapy selection and enhanced clinical care for cancer patients. According to the National Brain Tumor Society, more than any other cancer, brain tumors can have lasting and life-altering physical, cognitive, and psychological impacts on a patient’s life. Despite years of research, brain cancer survival rates have remained little-changed in recent years, even while survival rates for many other cancers have significantly improved.
Helge Lubenow, CEO of Heidelberg Epignostix, states, “The Epignostix CNS Tumor Methylation Classifier represents the only test of its kind in the market. We are incredibly pleased that CMS has released a Medicare rate for our CPT code for the Epignostix CNS Tumor Methylation Classifier, recognizing its value in improving cancer patient management”. She further notes, “This important reimbursement milestone will simplify the process and remove unknowns for molecular pathology laboratories in submitting claims and securing reimbursement”.
There are more than 180 distinct types of primary brain tumors, each with its own spectrum of presentations, treatments, and outcomes. Some are very difficult or impossible to diagnose without DNA methylation analysis.
Matija Snuderl, co-founder of Epignostix and Director of Molecular Pathology at New York Langone Health Hospital
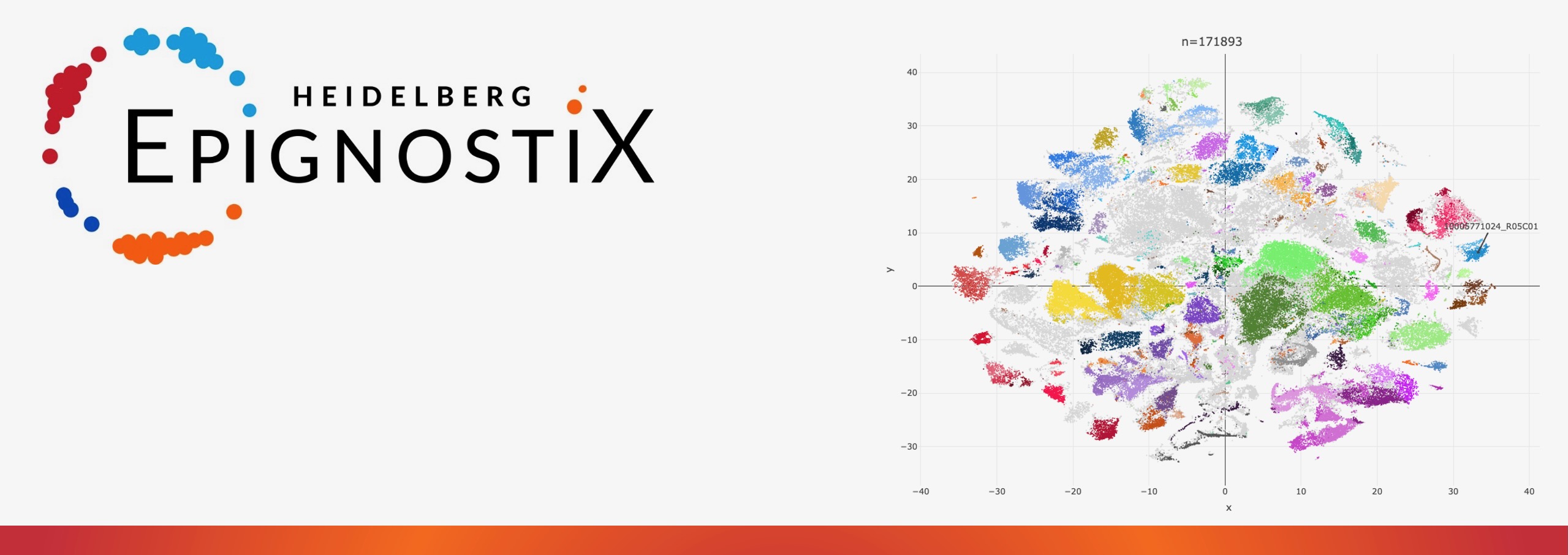
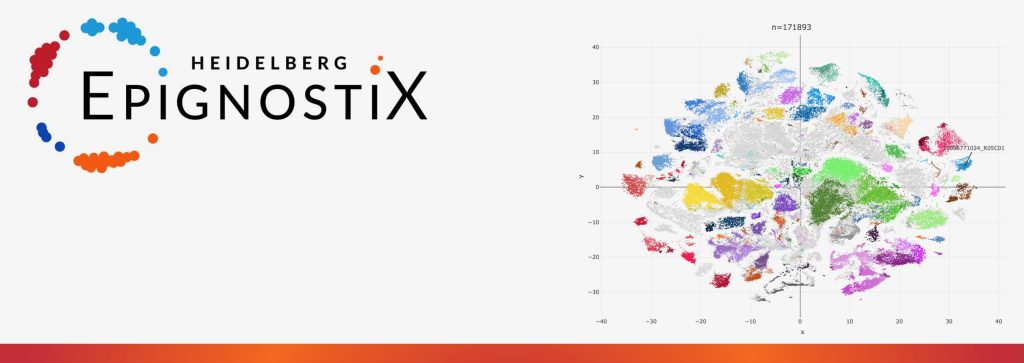
David Jones, CSO of Heidelberg Epignostix adds further: “Combining epigenetics with the power of artificial intelligence, our software algorithms enable robust and precise cancer classification. The technology is based on patterns of chemical tags on tumor DNA, DNA methylation, functioning as genomic fingerprints of the cancer’s identity. It has been applied to more than 185,000 samples to date.”
About Heidelberg Epignostix GmbH:
Heidelberg Epignostix is a healthcare company whose mission is to transform cancer diagnostics through data science. The company has developed novel AI-based bioinformatics algorithms with utility in cancer classification that hold promise for improved accuracy in cancer diagnosis, prognosis and therapy management. The lead product, the Heidelberg Brain Tumor Classifier, is currently available as a research-use-only resource. Heidelberg Epignostix is located in Heidelberg, Germany. For more information visit www.epignostix.com.
For further inquiries:
Areeba Patel
Heidelberg Epignostix GmbH
Nikola-Tesla-Straße
69124 Heidelberg
Germany
Email: areeba.patel@epignostix.com
www.epignostix.com